The Australian Breast Cancer Family Registry (ABCFR) is a resource of families, data, bio-specimens, researchers and community representatives established for the conduct of collaborative research on breast cancer. It is part of an international registry set up in the 1990s by the National Cancer Institute (NCI USA). Researchers from the USA, Canada and Australia have recruited volunteer families into six registries using common questionnaires and protocols. Data are collated at a centralised Informatics Support Center. We have collected epidemiologic risk factor and family history data for 8,700 participants from 2,200 families. We have collected 4,700 blood samples. Tumour material has been collected and the Breast CFR Pathology review has been conducted for 1,000 cases. Almost 50,000 DNA samples have been shipped to researchers.
The 10-year follow-up of The ABCFR
People
- More than 1,600 women diagnosed with breast cancer from 1992 to 2000, identified through the Victorian and New South Wales Cancer registries
- 1,000 women without breast cancer identified using electoral rolls
- 9,300 adult relatives (both male and female) of these women
- More than 400 women of Jewish descent who either have had breast cancer or have a relative who has had breast cancer
- Over 500 people from 70 families in which there are three or more women who have had breast or ovarian cancer, identified through cancer family clinics
- 65 twin pairs in which one or both have had breast cancer, identified through the Australian Twin Registry, and over 300 of their relatives
- 120 women recruited in 2011 (65 of which were diagnosed with breast cancer in 2009, and identified through the Victorian Cancer registry)
Resource
- 3,317 families
- 13,363 baseline epidemiology questionnaires
- 8,816 blood samples
- 295 BRCA1 and BRCA2 carriers
- 818 women with digitized mammograms
The 10 year follow-up
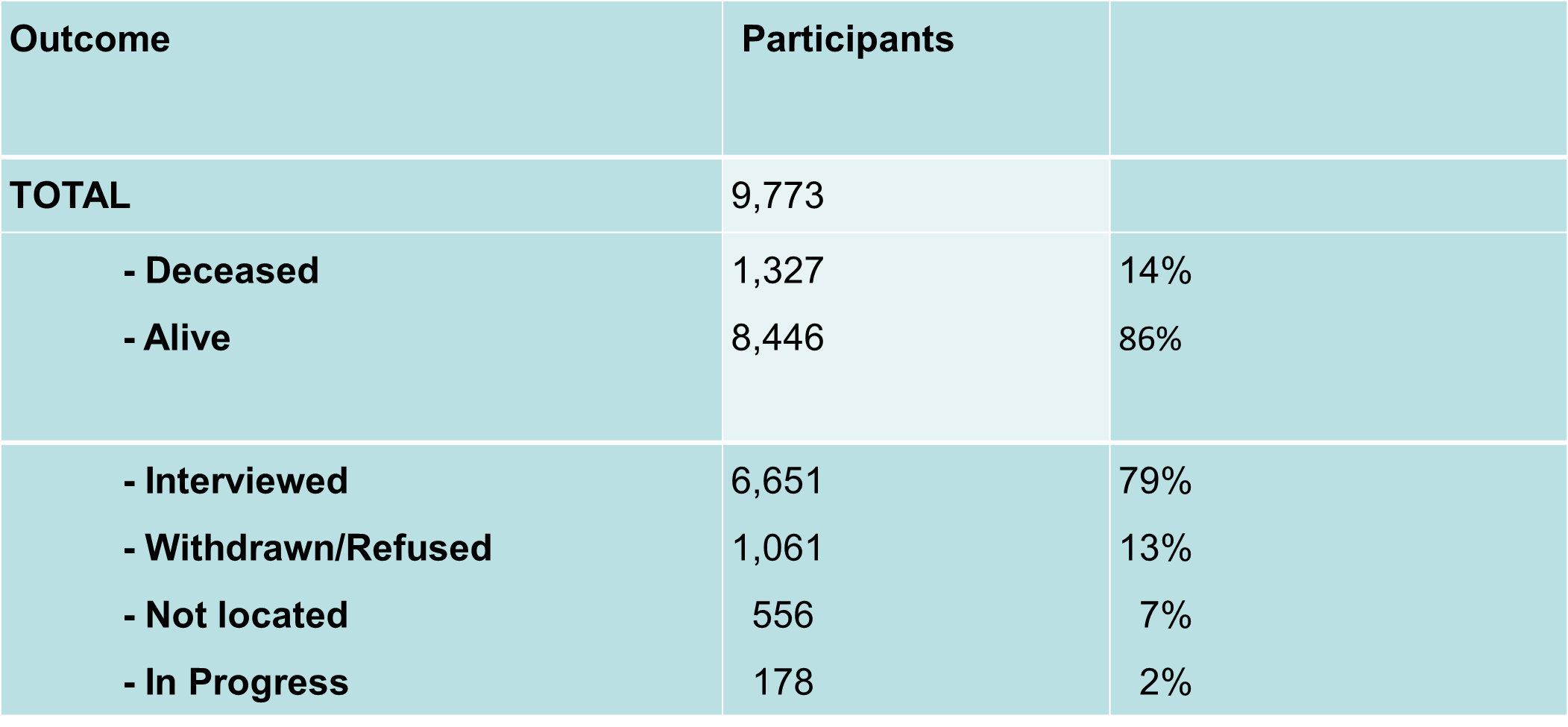
The 9,973 participants from 22,72 families who were informed at baseline interview that we may wish to contact them in the future were eligible for the 10-year follow-up of the ABCFR.
2003 to 2006: 1,422 participants from 248 ABCFR families recruited from 1992 to 1995 were followed up, funded by Cancer Australia, NBCF and NHMRC.
2007 to 2012: 8,351 women and men from 2,024 families recruited to the ABCFR from 1996 to 2000 were followed up. At the same time, a further 17,000 women and men were followed up at the five North American sites of the Breast Cancer Family Registry., funded by NIH (USA) All six sites (ABCFR and the North American sites) used the same questionnaires.
An invitation letter with information sheet, consent form and short questionnaire was mailed. This was followed up with a phone call to expand on responses to the questionnaire and obtain a family history from the participant and other data as required.
Additions to the ABCFR at 10-year follow-up
- Updated family history for 1,982 (87%) families
- Updated epidemiology data for 6,651 (79%) participants
- 1,759 additional blood samples collected
- 196 additional BRCA1 and BRCA2 clinical genetic testing reports received
- 694 new participants recruited
- 360 new breast cancer cases identified
Table 1. New breast cancers in ABCFR participants at 10-year follow-up
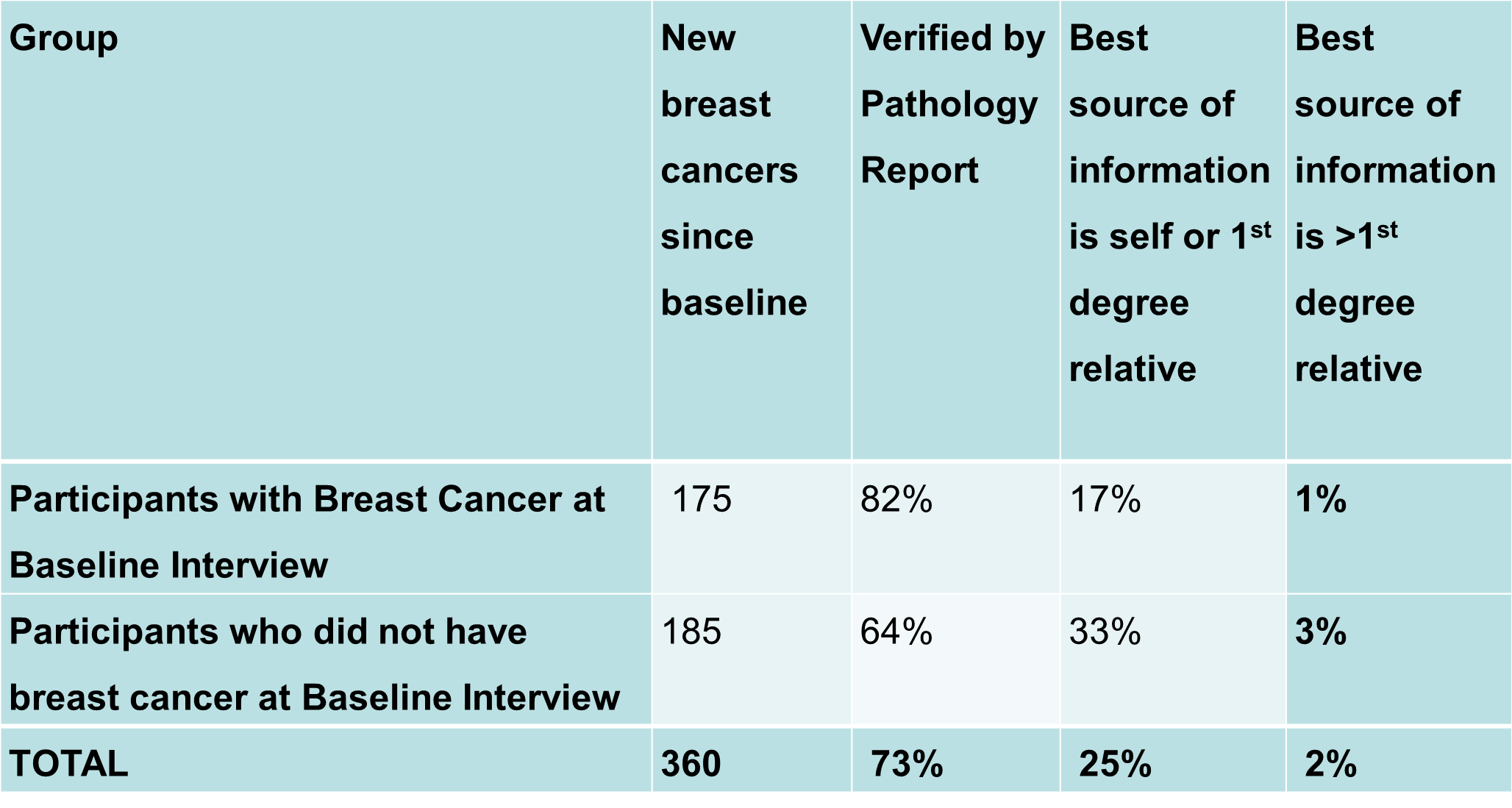
Table 2. Epidemiology data update in the ABCFR at 10-year follow-up
Pedigrees no longer proband -centric
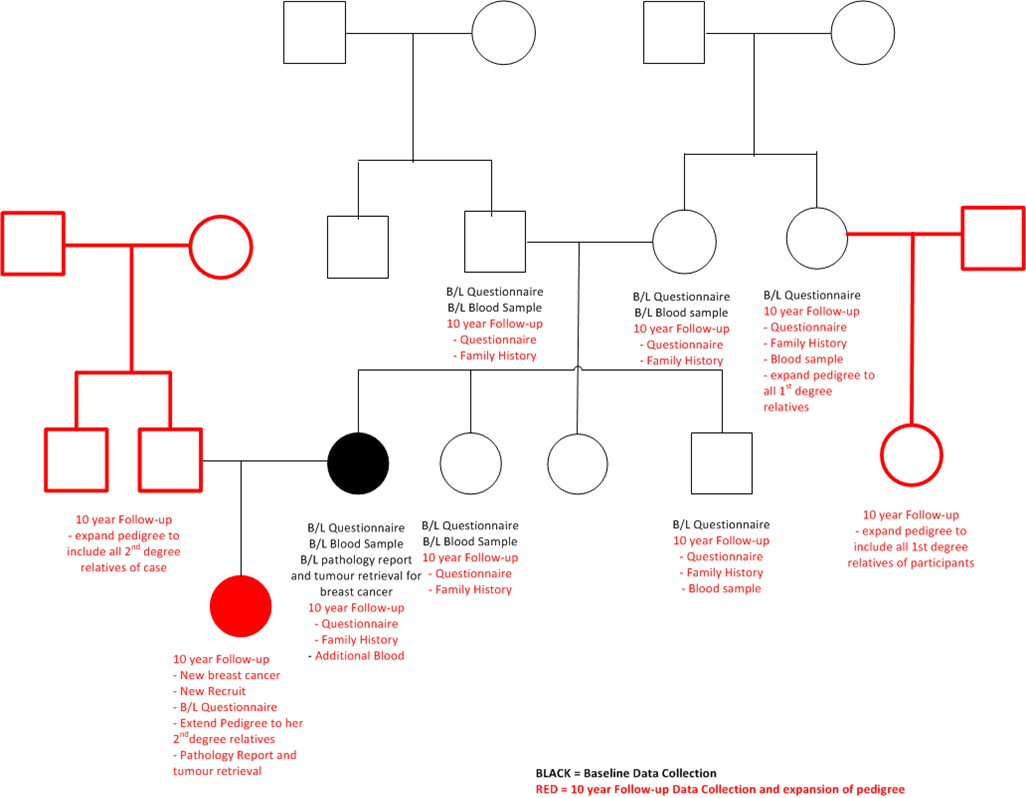
Multiple reporting at 10-year follow-up for the BEST ESTIMATE of Family History
To minimise reliance on reports from 2 nd degree or more distant relatives, EVERY participant was asked their personal and family cancer history, up to their 2nd degree relatives. If there remained individuals on the pedigree that had not been updated, key family members were asked to update the remaining details.
This resulted in MULTIPLE REPORTS from the participants on their family members.
The rules for updating the family history were:
- Verification, if available* > self > 1st degree > 2nd degree and so on
- Interviewer can, with justification, accept a report from a more distant relative over a closer relative if he/she thinks they are more ‘reliable' (interviewer judgement)
- Where discrepancies occur across equal sources (for example, 3 sisters reported different cancer details about their mother), the interviewer who spoke to the family, with the assistance of the supervisor if required, used their best judgement about which report(s) to accept.
*Cancer verifications were obtained from the CCV, via pathology report and we are currently conducting linkage with the AIHW for verification of cancers Australia-wide.
Prospective family study cohort ( ProF -SC) designs involving people across a range of familial risk profile provide a resource for epidemiological, genetic, behavioural, psycho-social and health utilisation research. The prospective aspect gives credibility to risk estimates. The familial aspect allows family-based designs, matching for unmeasured factors, adjusting for underlying familial risk profile and, as shown here, enhanced cohort maintenance.
Reference: Hopper JL. Disease-specific prospective family study cohorts enriched for familial risk. Epidemiol Perspect Innov. 2011 Feb 27;8(1):2