The Australasian Colorectal Cancer Family Register is Australia's foremost resource for research into the genetic and environmental causes of bowel cancer. The Principal Investigator, Prof John Hopper in collaboration with Prof Jeremy Jass and colleagues throughout Australia and New Zealand, has been awarded $16.7m over 10 years to recruit colorectal cancer families, and collect blood samples, epidemiological questionnaires, tumour samples, medical records, dietary questionnaires and family cancer histories from more than 28,000 individuals from over 7,400 families. This study is funded by the National Institutes of Health (USA) as part of an international consortium, the Colon Cancer Family Registry. Other collaborating institutions include the Mayo Clinic, the Fred Hutchinson Cancer Research Center, the University of Hawaii, Cancer Care Ontario, and the University of Southern California. More than 13,000 families have been recruited. Major funded projects stemming from the Colon CFR include: studies to identify new genes for bowel cancer; studies on bowel cancer aetiology including molecular pathways; studies on the risk of cancer in carriers of mutations in known high risk genes; and studies on cancer prevention including screening - as well as over 100 other studies related to bowel cancer.
BACKGROUND
-
The Australasian Colorectal Cancer Family Registry (ACCFR) was established in 1997 and has been recruiting, surveying, collecting biospecimens, following-up, and genetically characterising for the past 15 years.
-
It constitutes one of the six registries of the NIH funded international Colon Cancer Family Registry that was established as a resource for research into the genetic and environmental aetiology of colorectal cancer.
RECRUITMENT
Family Cancer Clinics
Probands: Attendees to family cancer clinics with a family history of colorectal cancer, or being a member of a family known or suspected to be segregating a mutation in a mismatch repair or an MUTYH gene.
Relatives: Probands and other participants were asked for permission to recruit first- second-degree relatives of proband and additional family members of proband.
Community
Probands: Self- nominated due to promotion or advertisement of ACCFR
Relatives: Probands and other participants were asked for permission to recruit first- second- and additional family members of proband.
Population-based
Case probands: incident first primary colorectal cancer diagnosed in metropolitan Melbourne between 1996 and 2008 before age 50 years (100% attempted), or between age 51 and 60 (50% attempted).
Control probands: Age- and sex-matched to cases from the electoral roll
Relatives: Probands and other participants were asked for permission to recruit first- second-degree relatives of proband and any first-degree relatives of colorectal cancer affected participants (sequential ascertainment).
Back to top
BASELINE PROTOCOL
-
Interviews were administered in-person or by telephone for all participants. Questionnaires were completed for lifestyle factors, family history of cancer and diet (see below).
-
Blood samples (40 mls) were requested from selected participants.
-
Tumour samples were sought from pathology laboratories and hospitals for all reported CRC and other Lynch syndrome cancers.
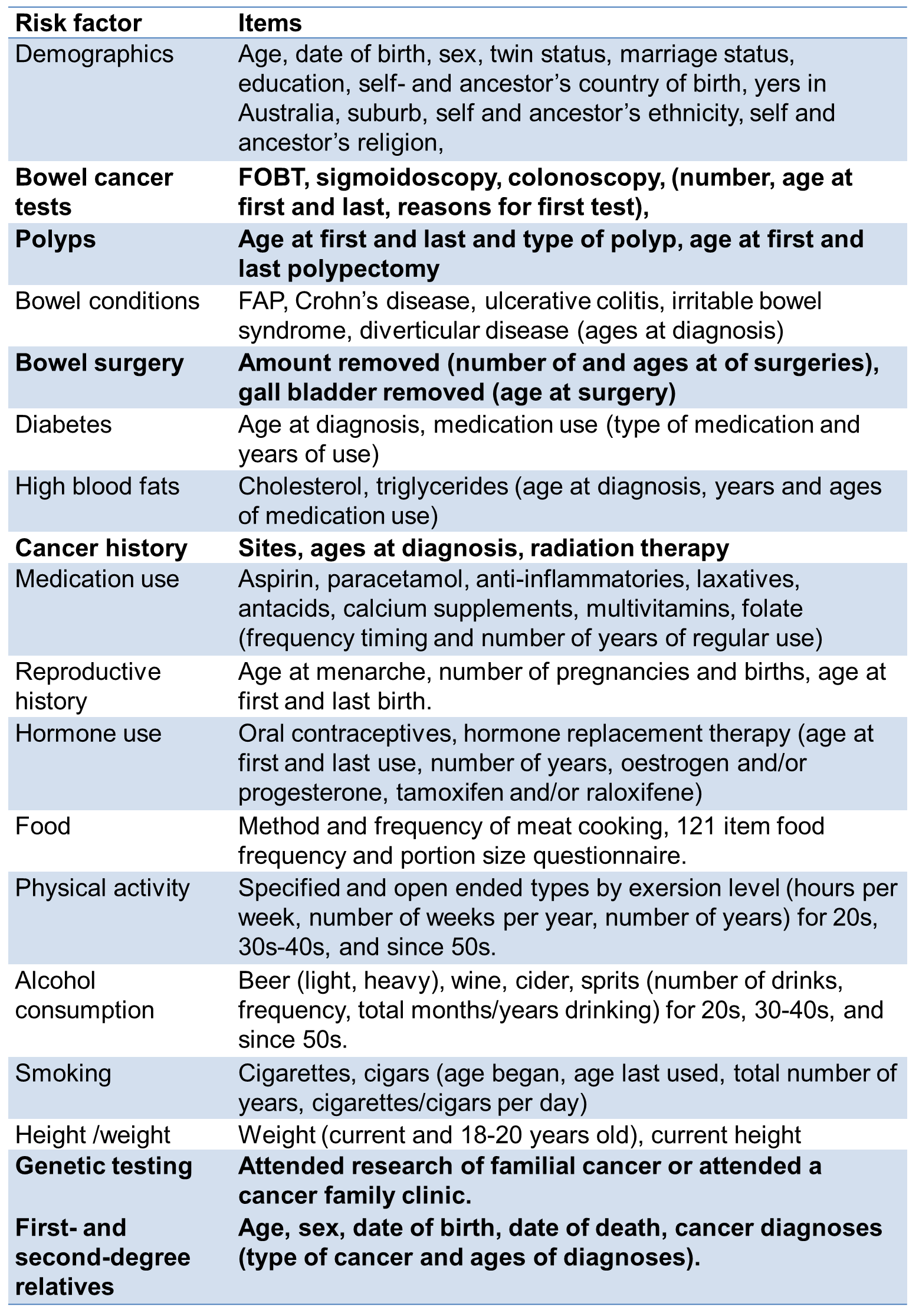
Note: Bold items used in follow-up.
Back to top
RESOURCE
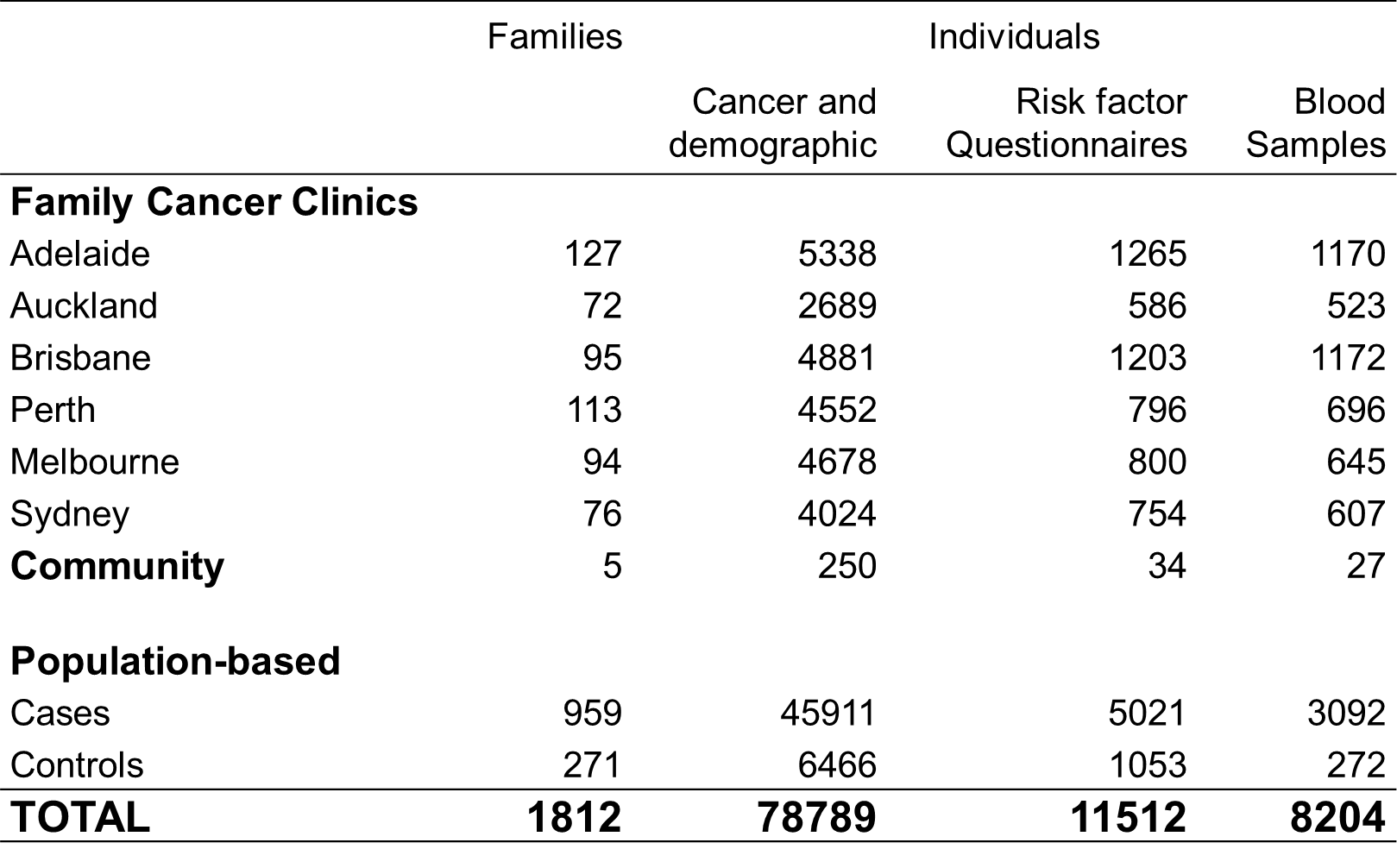
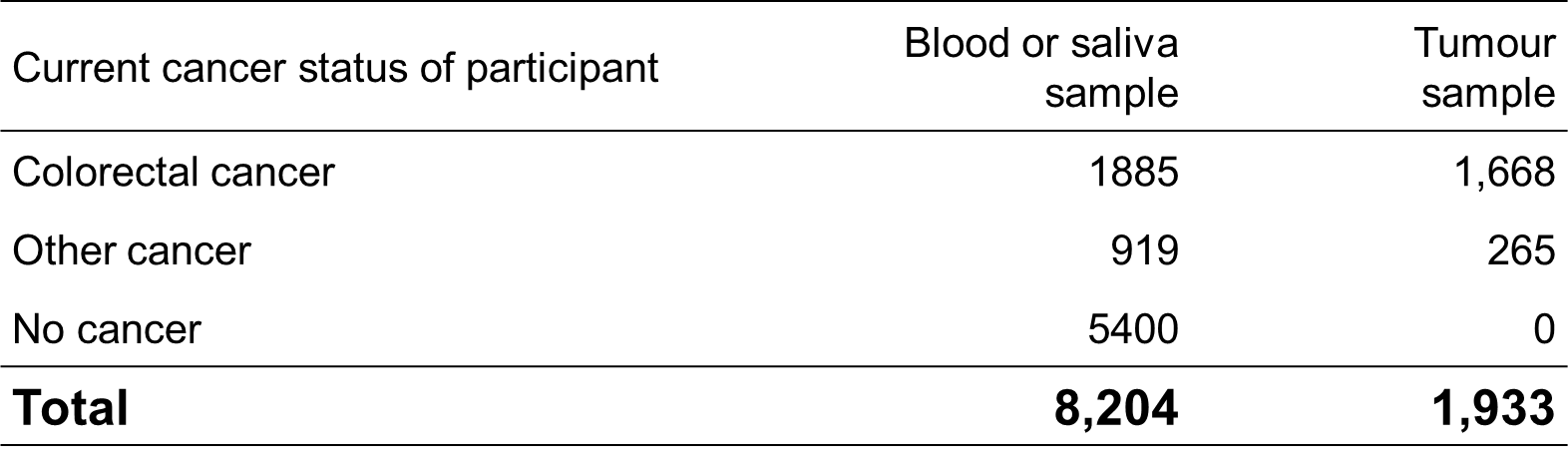
Back to top
MOLECULAR CHARACTERISATION
Mismatch repair gene mutation testing
-
Performed by Sanger sequencing or denaturing high performance liquid chromatography, followed by confirmatory DNA sequencing.
-
Large duplication and deletion mutations were detected by Multiplex Ligation Dependent Probe Amplification.
-
A pathogenic mutation was defined as a variant that was predicted to result in a stop codon, a frameshift mutation, a large duplication or deletion, or a missense mutation previously reported within scientific literature and databases to be pathogenic.
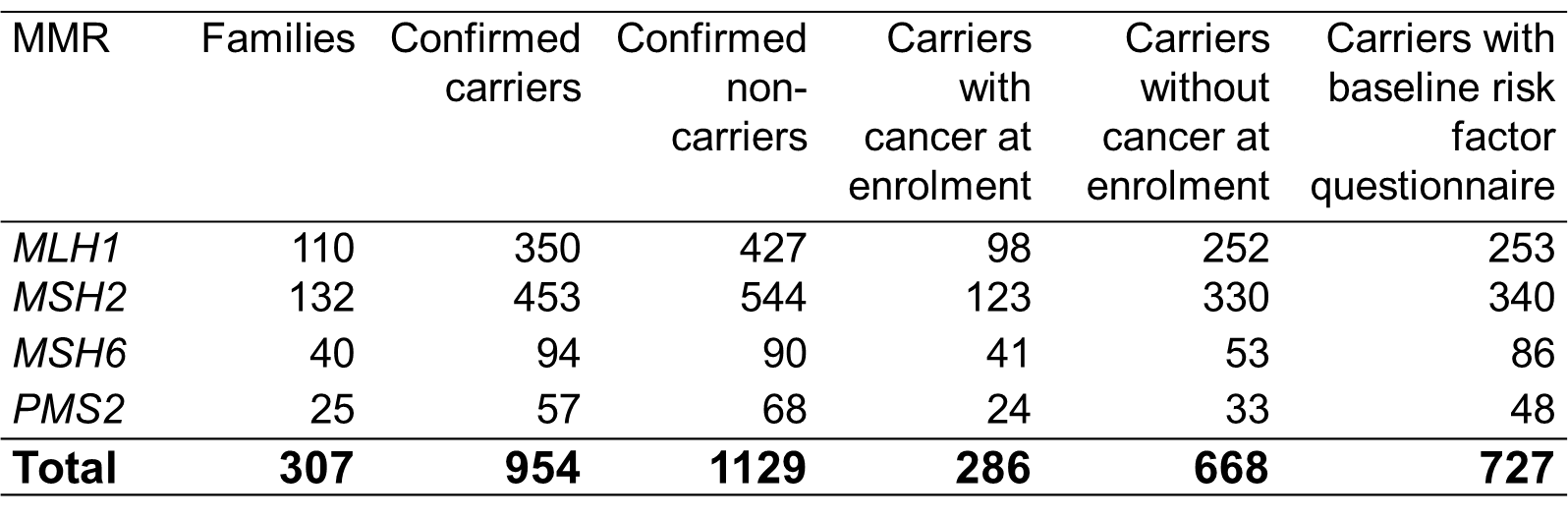
MUTYH gene mutation testing
-
Genomic DNA extracted from each participant was sent to a central testing facility (Analytic Genetics Technology Centre, Toronto, Canada).
-
DNA was screened for 9 variants of MUTYH mutations: Y179C, G396D, Y104X, R274Q, E480X, Q391X, c.1147delC, c.933+3A>C, and c.1437_1439delGGA, using the MassArray MALDI-TOF Mass Spectrometry (MS) system (Sequenom, San Diego, CA).
-
All samples with MS mobility shifts underwent screening of the entire MUTYH coding region, promoter, and splice sites regions by denaturing high-performance liquid chromatography (Transgenomic Wave 3500HT System; Transgenomic, Omaha, NE), to confirm the mutation and to identify additional mutations.
-
All MS-detected variants and WAVE mobility shifts were submitted for sequencing for mutation confirmation (ABI PRISM 3130XL Genetic Analyser).
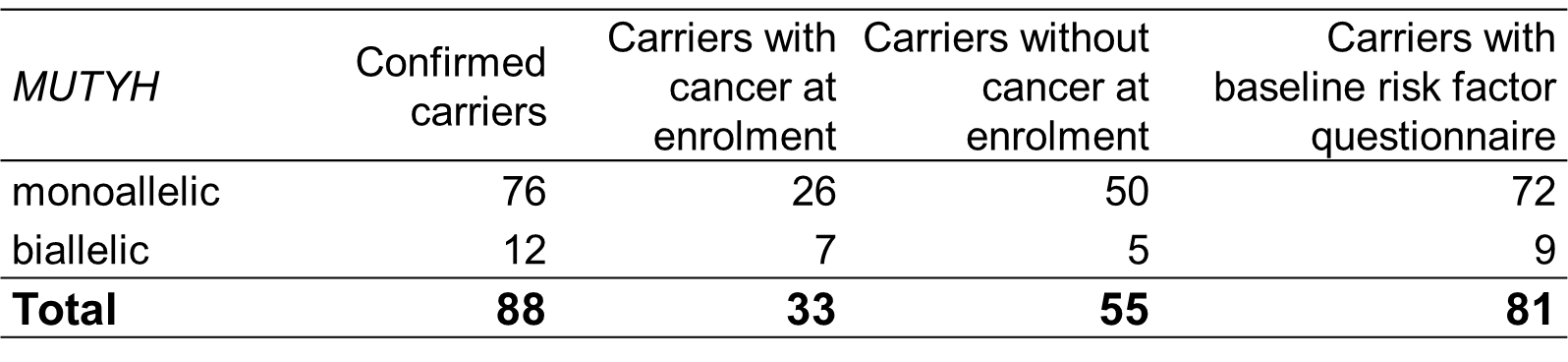
Tumour characterisation
-
Primary CRC tissue from the ACCFR Jeremy Jass Memorial Tissue Bank underwent pathology review and molecular characterisation as follows.
-
CRCs from the probands were characterised for MMR-deficiency by:
-
microsatellite instability testing (MSI) using a ten-marker panel where tumours with 30% or more of the markers show instability were considered to have high levels of microsatellite instability (MSI-H) and
-
by immunohistochemistry (IHC) for the expression of the four MMR proteins.
-
CRCs demonstrating loss of expression of the MLH1 and PMS2 proteins by IHC were subsequently characterised for methylation of the MLH1 gene promoter using a MethyLight assay.
-
All proband CRCs were tested for the BRAF p.V600E somatic mutation using allele-specific PCR.
-
All CRCs were reviewed by specialist GI pathologists for tumour site, grade, margin, presence of mucinous component, peritumoral lymphocytes, Crohn's-like lymphocytic reaction, tumour-infiltrating lymphocytes, and synchronous CRC.

Back to top
FOLLOW-UP
Active Follow-up
-
Follow-up of all families (except population-based control families) attempted approximately every 5-years.
-
A mixture of methods was used. Participants were asked to complete a telephone interview or were mailed a questionnaire to complete with phone follow-up for additional information and for family history of cancer.
-
The mailed questionnaire was abbreviated to ask major details on cancer diagnoses, screening, surgery, family history and genetic testing.
-
Medical records were sought to verify all reported CRC and other Lynch syndrome cancer. On average, 110 reported cancer were verified per year.
-
Medical records were sought to verify all reported polyps. On average, 108 reported polyps were verified per year.
-
Blood samples were requested for participants with new diagnoses of CRC and other Lynch syndrome cancers, and new recruits in existing families, and for whom DNA samples needed replenishing. On average, 210 blood samples were collected each year of follow-up.
Passive Follow-up
-
All family members (participants and non-participants) were linked to the National Death Index to verify vital status and causes of death.
-
All family members (participants and non-participants) who lived in Victoria were linked to the Victorian Cancer Registry to verify and update cancer diagnoses and vital status.
-
All family members (participants and non-participants) currently being linked to the National Cancer Clearing House to verify and update cancer diagnoses.
Back to top
PROGRESS

Note: This does not include population-based control families
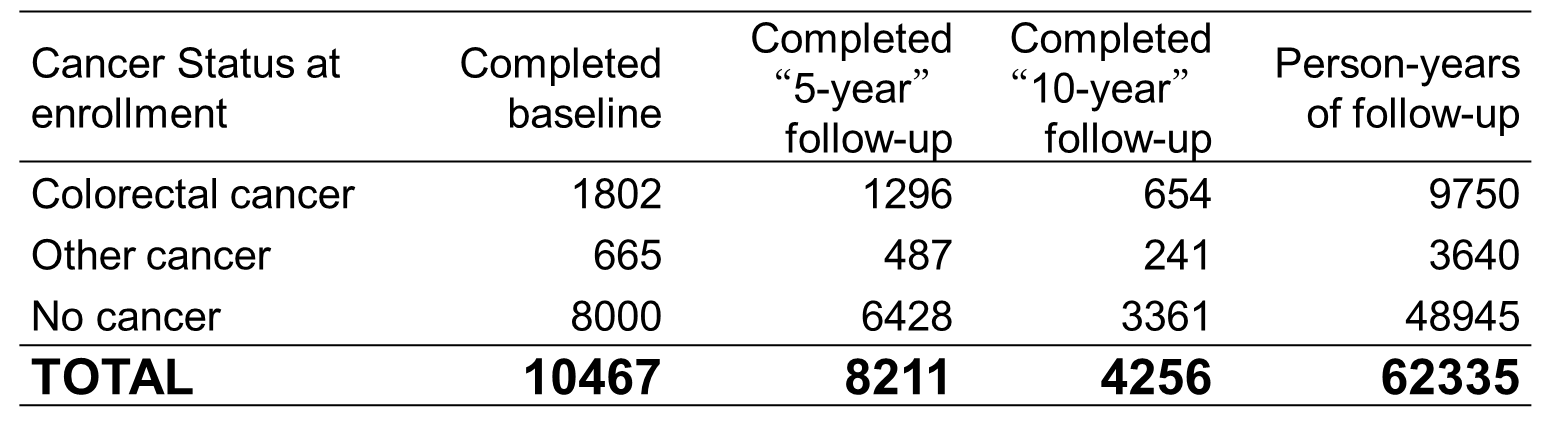
Note: This does not include population-based control families
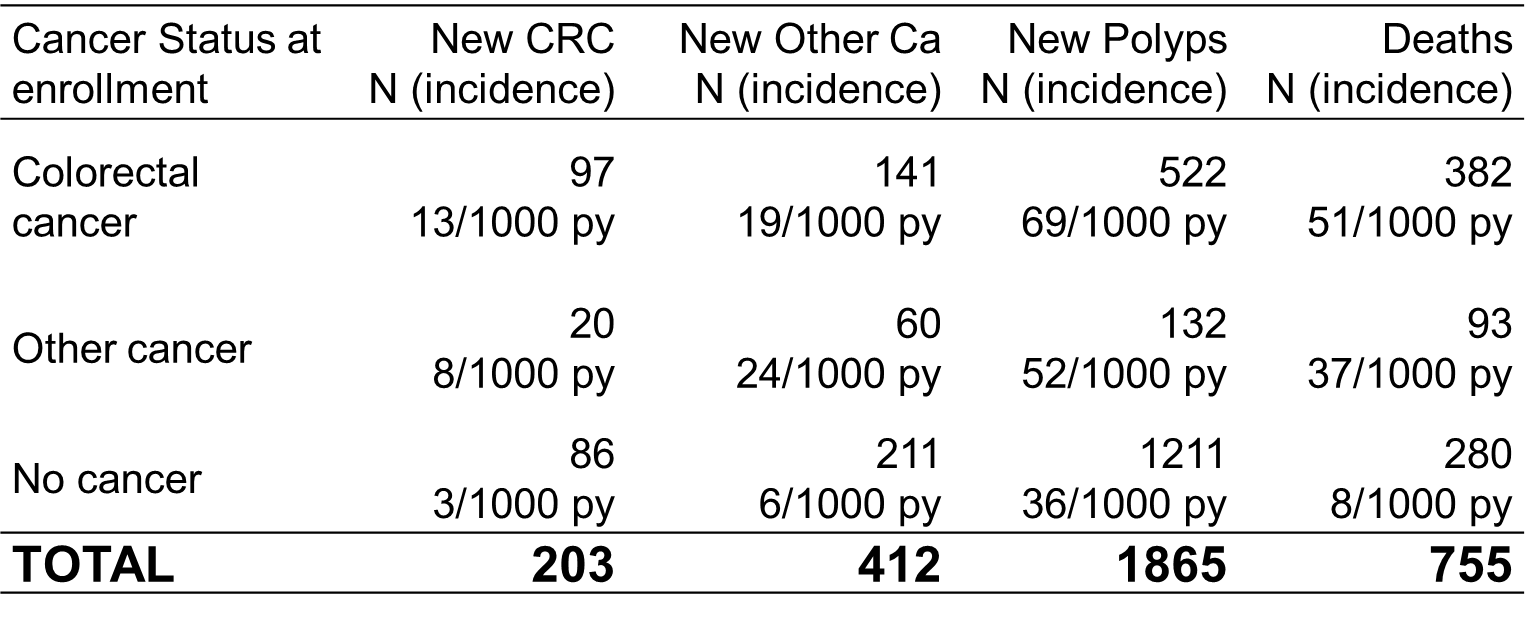
Back to top
FEEDBACK OF TEST RESULTS
-
Participants of MMR families detected by ACCFR are given the opportunity to learn of their genetic status via a clinical genetics service.
-
The ACCFR has accordingly written to 1622 participants of 330 families segregating a mutation in a mismatch repair gene or an MUTYH gene.
-
The ACCFR follow the progress of participants through the counselling process to determine how many attend a clinic and learn of their results.
CONCLUSIONS
-
The ACCFR is the largest and best characterised colorectal cancer family registry in the world and has been a major contributor to colorectal cancer research through the Colon Cancer Family Registry.
-
We have demonstrated that, using a prospective family-study cohort design, we can achieve very high response over a decade of follow-up.
ACKNOWLEDGEMENTS
-
National Cancer Institute, National Institutes of Health under RFA #CA-95-011 (Colon CFR)
-
Picchi Brothers Foundation Cancer Council Victoria Postgraduate Cancer Research Scholarship (AKW)
-
NHMRC Senior Research Fellow (MJ, MS), Australia Fellow (JH)
Back to top
CONTACT
Mark A. Jenkins, PhD
Principal Investigator
Centre for MEGA Epidemiology
Melbourne School of Population Health
The University of Melbourne
207 Bouverie Street,
The University of Melbourne
Victoria 3010, Australia
Ph: +613 8344 0902
Email: m.jenkins@unimelb.edu.au